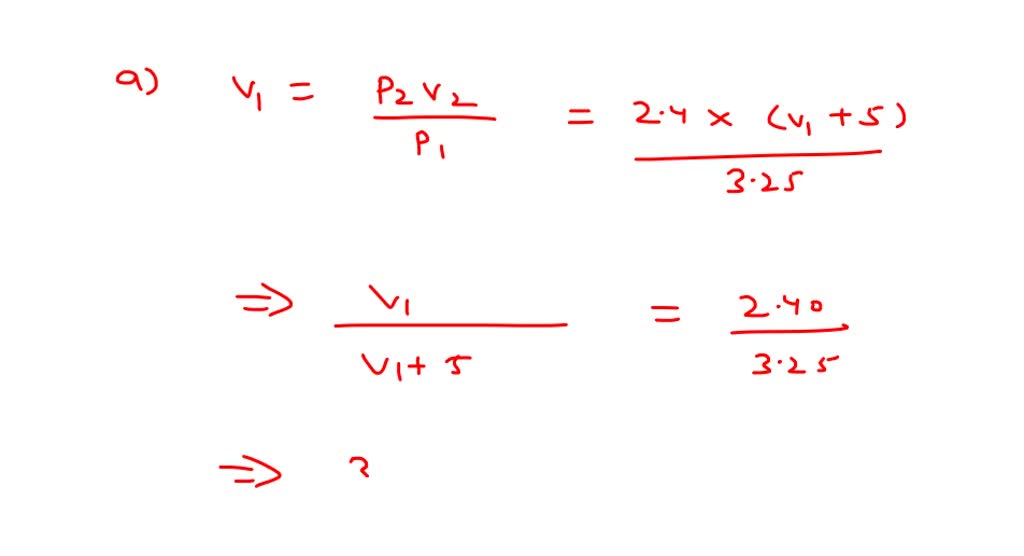1.00 g of water is introduced into a 5.00 L evacuated flask at `50^@C`. `{:("Vapour","Pressure at - YouTube
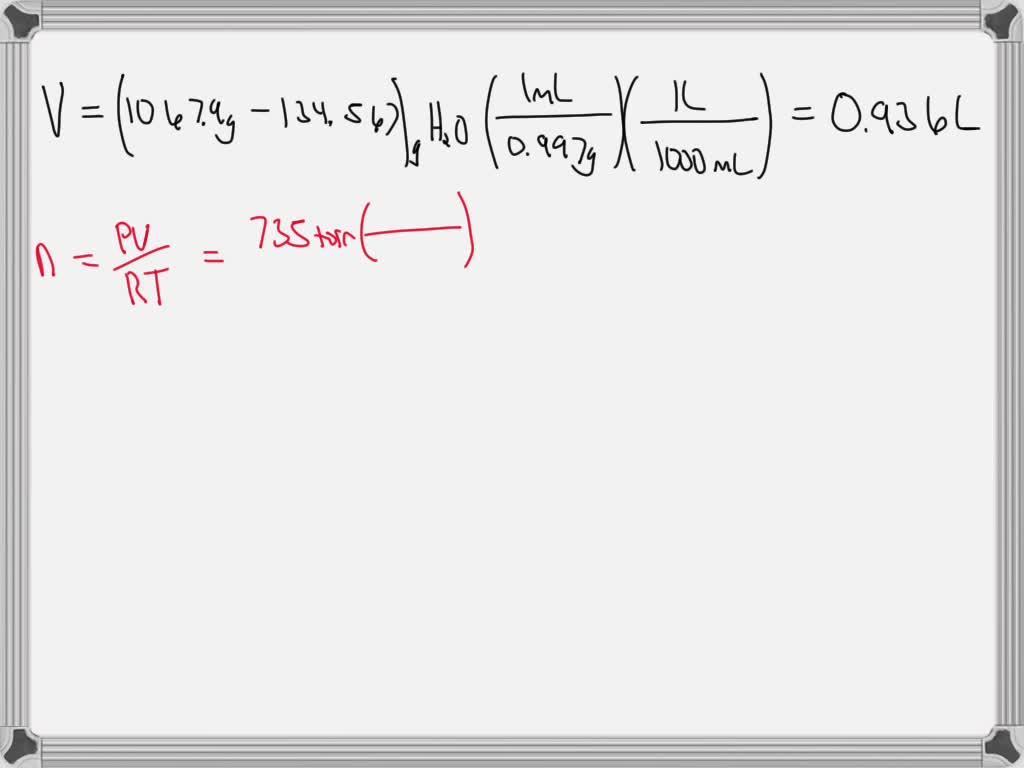
SOLVED: A large evacuated flask initially has a mass of 134.567 g. When the flask is filled with a gas of unknown molar mass to a pressure of 735 torr at 31

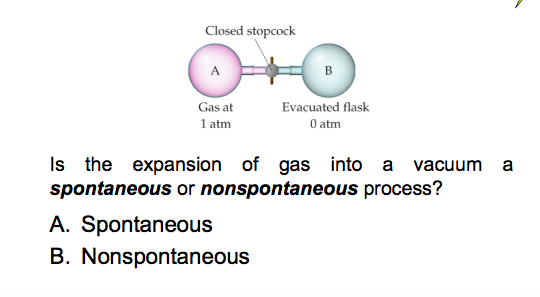
When 2 g of gas A is introduced into an evacuated flask kept at 25^oC , the pressure was found to be 1 atm. If 3 g of another gas B is

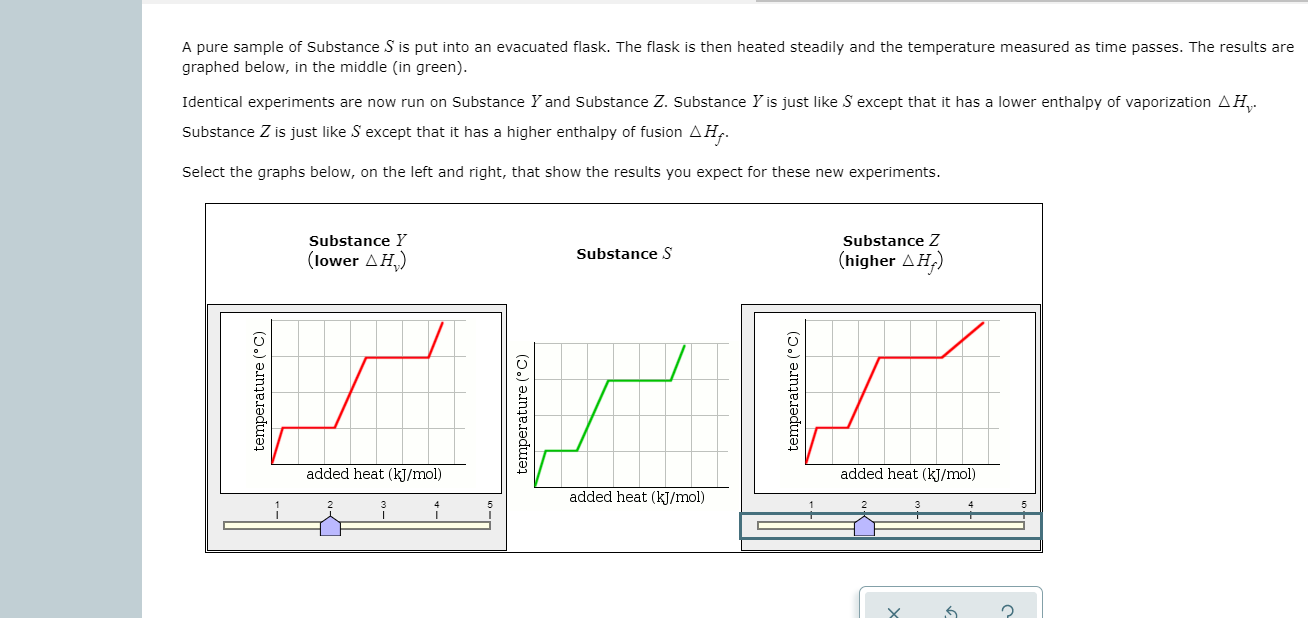
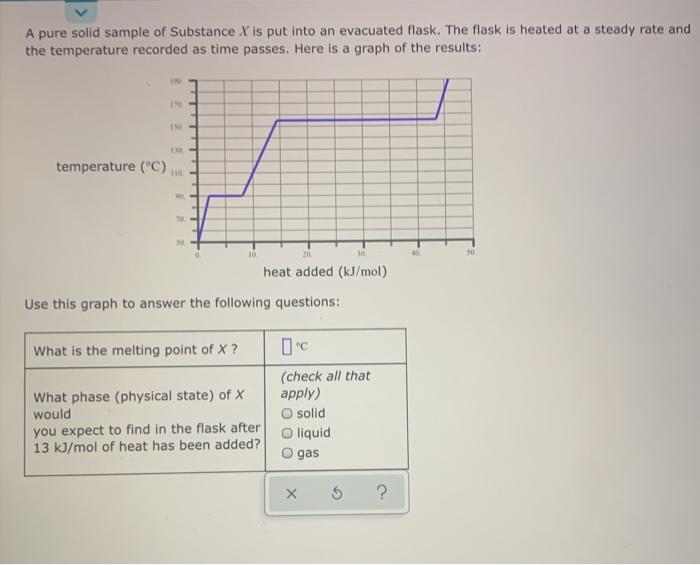
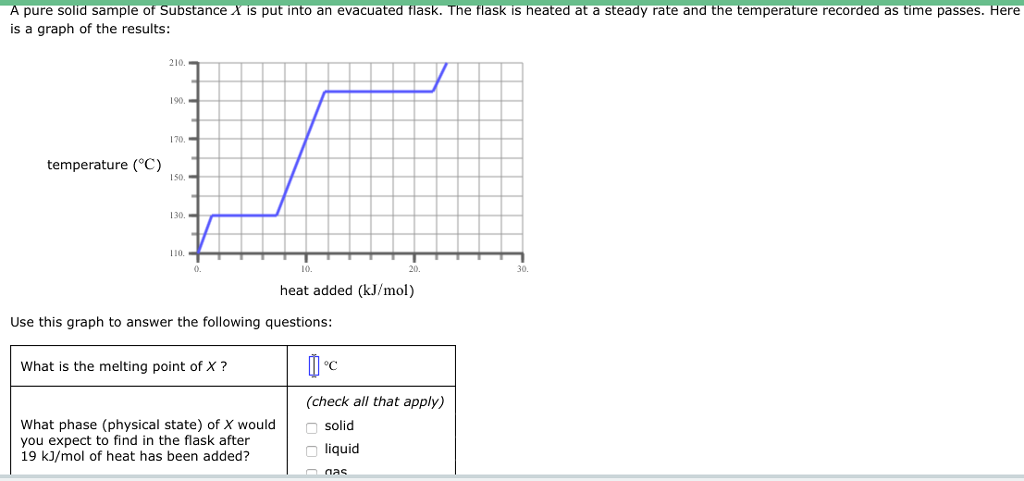
SOLVED: pure sample of Substance S is put into an evacuated flask: The flask is then heated steadily and the temperature measured as time passes The results are graphed below, in the

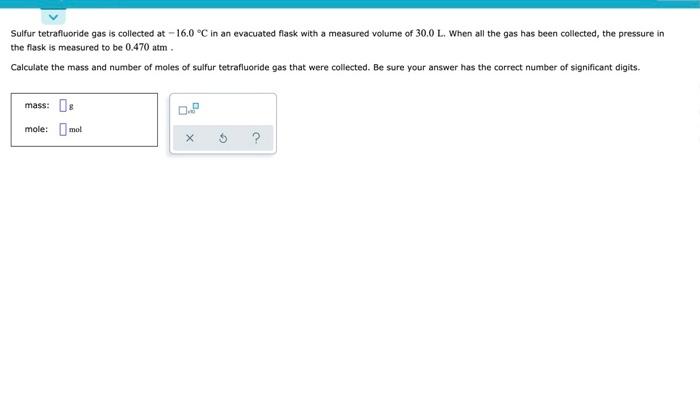
Dinitrogen monoxide gas is collected at -20.0°C in an evacuated flask with a measured volume of 7.0 - YouTube

When 2 g of a gas A is introduced into an evacuated flask kept at 25^(@)C, the pressure is found... - YouTube